| Case Report Online Published: 13 Jul 2023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sudan J Paed. 2023; 23(1): 98-103 SUDANESE JOURNAL OF PAEDIATRICS 2023; Vol 23, Issue No. 1 CASE REPORT Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese childOmer S. M. Suliman (1)Paediatric Department, Faculty of Medicine, University of Khartoum, Khartoum, Sudan Correspondence to: Omer S. M. Suliman Department of Paediatrics, Faculty of Medicine, University of Khartoum, Khartoum, Sudan. Email: omer.suli1959 [at] gmail.com Received: 17 December 2021 | Accepted: 21 February 2023 How to cite this article: Suliman OSM. Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudan J Paediatr. 2023;23(1):98–103. http://doi.org/10.24911/SJP.106-1639730602 © 2023 SUDANESE JOURNAL OF PAEDIATRICS
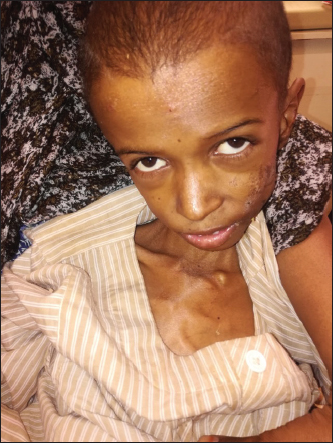
ABSTRACTThe prevalence of coeliac disease (CD) is rapidly rising in both developed and underdeveloped countries. CD classically presents with gastrointestinal manifestations, but it is now increasingly considered as a multisystem disease mostly affecting the central nervous system. Recently, a non-biopsy approach for the diagnosis of CD has been recommended by the European Society for paediatric gastroentrology, hepatology and nutrition. Here, we are reporting a 12-year-old Sudanese boy who presented with chronic diarrhoea and weight loss and lower limbs weakness. His examinations showed emaciation, pallor and weakness of both lower limbs and mixed upper and lower motor neuron signs and peripheral neuropathy, suggestive of sub-acute combined degeneration of the spinal cord (SACDSC). His initial investigations showed microcytic hypochromic anaemia with hypokalaemia and hypocalcaemia and very high titer of the IgA class of tissue transglutaminase (28× upper limit normal ) with a positive anti-endomeseal IgA antibodies. He was diagnosed with acute coeliac crisis with SACDSC, most likely due to Vitamin B12 deficiency. Although his initial cobalamine level was normal, he later developed macrocytosis and his neurological signs improved rapidly with injectable B12. We reported a rare neurological presentation of CD and we highlighted the non-biopsy approach for the diagnosis of CD in children. KEYWORDS:Celiac disease; Sub-acute degeneration of spinal cord; Child. INTRODUCTIONBesides coeliac disease (CD), the spectrum of gluten-related disorders includes dermatitis herpetiformis, gluten-sensitive ataxia and non-coeliac gluten sensitivity [1] CD is a chronic multi-organ autoimmune disease that affects the small bowl in genetically predisposed persons precipitated by the ingestion of gluten [2]. A subgroup of CD is regarded as a potential CD because they have a normal small bowel mucosa, but positive CD serology along with human leukocyte antigen (HLA) DQ8 and/or DQ2 positivity [3]. Depending on certain clinical, immunological and histopathological characteristics, CD may be subdivided into different categories, such as seronegative, slow responders and refractory CD [4]. The prevalence of CD is rapidly rising in both developed and underdeveloped countries and it ranges between 1% and 0.6% [5]. Classically, CD presents with gastro-intestinal manifestations such as diarrhoea, abdominal pain and weight loss [6]. It is now increasingly been considered a multi-organ disease with extra-intestinal manifestations, including neurological diseases [7]. In this case report, we present a Sudanese child who presented with a rare neurological manifestation of CD, which is sub-acute combined degeneration of spinal cord (SACDSC). Another aim was to highlight the recently published European society for paediatric gastroentrology, hepatology and nutrition (ESPGHAN) guidelines, the no biopsy approach, for the diagnosis of CD in children. CASE REPORTA 12-year-old Sudanese boy was admitted to the general paediatric ward at Ibrahim Malik Teaching Hospital, Khartoum, Sudan, on 10/30/2020 with a history of profuse watery diarrhoea and frequent vomiting for the last 10 days. During the last 3 years, he had recurrent attacks of similar complaints for which he was admitted several times to different hospitals and managed as acute gastroenteritis. The diarrhoea was always watery and never contains any blood or mucous. However, it was offensive and associated with occasional vomiting and abdominal distension. He had marked weight loss despite a good appetite. He had no history of fever, cough or shortness of breathing. One month prior to admission, he developed pain and heaviness in both lower limbs and tingling sensations on both feet. These were followed by a progressive weakness of both legs and he became completely bedridden for the last 10 days. His birth and developmental histories were normal. Parents are first-degree cousins but have no family history of CD or any other autoimmune disease. He was exclusively breast fed for 6 months and complementary feeding started at 8 months with no ill effects. He had a history of pica and was noticed to be pale and diagnosed with iron deficiency anaemia, which did not respond to many courses of oral iron therapy. He has no history of blood transfusion. However, he received many antibiotics for the treatment of the diarrhoea. On clinical examination: he was found to be ill and pale with signs of dehydration but with stable vital signs. Further evaluation revealed severe wasting (Figure 1), both weight (15 kg) and height (129 cm) were below the third centiles. His abdomen was distended but soft with no clinical signs of ascites. Examination of the cardiovascular, chest, skin and joints was unremarkable. Neurological examination showed an apathetic but fully oriented boy. Cranial nerves including fundi were normal. Upper limbs showed mild weakness but with normal tone and reflexes. Lower limbs showed generalised weakness with power grade 3/5, hypotonia with absent deep tendon reflexes at knees and ankles but with a positive Babinski sign (Figure 2). He had an impaired position sense but with intact pinprick and fine touch and abdominal reflexes with no sensory level. The impression was SACDSC with acute coeliac crisis. His initial investigations showed microcytic hypochromic anaemia, hypokalaemia, hypo-albuminaemia and mildly raised liver transaminases with normal vitamin B12 level (Table 1). Coeliac serology showed very high anti-tissue transglutaminase (tTG) IgA levels (28 times the upper limit of normal [ULN], and a positive anti-endomyseal antibodies).
Figure 1. 12-year-old child with CD showing severe wasting and pallor.
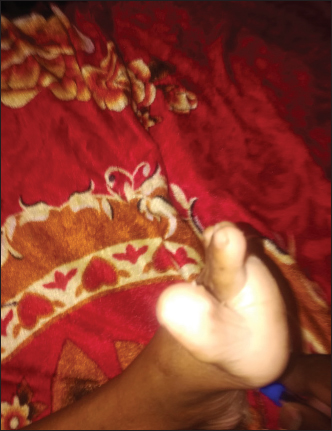
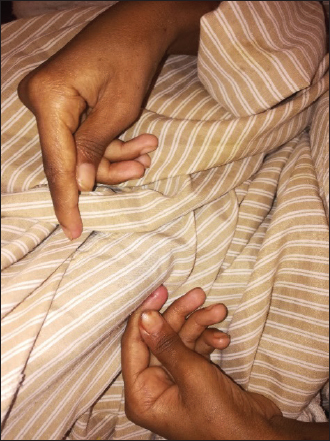
Figure 2. Positive Babinski sign. The child was urgently managed with fluid resuscitation with normal saline (NS) bolus, then for moderate dehydration with D51/2NS and given KCL infusion and started on intravenous methylprednisolone 2 mg/kg/day over 2 days then tapered with oral prednisolone over 2 weeks. He developed tetany with carpopedal spasm (Figure 3), and was found to have hypocalcaemia, which was corrected with calcium gluconate infusion. In addition, he was given intramuscular (IM) vitamin B12, folic acid, oral zinc, vitamin D3, oral copper and oral multivitamins with therapeutic dose of IM vitamin K. IM vitamin B12 was given daily for 1 week, then once per week for 1 month then every month for 3 months. The patient showed rapid improvement in his neurological signs; within 1 week, he started to sit on the bed, next week he was walking with a mild ataxic gait and was discharged home after 1 month with strict GFD and a follow-up with a dietician expert in CD management. Table 1. Laboratory values on admission and 2 weeks on gluten-free diet (GFD).
*Anti-tTG IgA was repeated after 1 month on GFD.
Figure 3. Carpal spams due to hypocalcaemia in a child with CD. On follow-up 1 month later, he gained significant weight with normal neurological examination and improvement in his anaemia, and with normal ant-tTG IgA level (Figure 4). DISCUSSIONThe case reported here showed the classic and a rather historical presentation of CD, with severe emaciation and acute coeliac crises. Yet it represents a delayed diagnosis, despite the availability of non-invasive and reliable serological tests. However, it also demonstrated the rapid and effective response to the GFD. Currently, CD can be considered into different categories, including classical CD, atypical CD, asymptomatic and latent CD [7]. Classic CD, including three features, symptoms of malabsorption, villous atrophy and resolution of symptoms on GFD. This variant usually presents with malabsorptive symptoms with vitamins and other micronutrient deficiencies. The malabsorptive symptoms include steatorrhea, weight loss, abdominal pain and distension, and diarrhoea. The micronutrient deficiencies include iron, folic acid, vitamin B12, and vitamins D and zinc, [8]. Our patent demonstrated most of these features like chronic and recurrent diarrhoea, weight loss, muscle wasting and persistent iron deficiency anaemia. However, this patient also showed signs of the SACDSC, involving the lower limbs with mixed upper and lower motor neuron signs and peripheral neuropathy. This could be due to vitamin B12 deficiency. Although his initial laboratory tests showed microcytic hypochromic anaemia with normal serum vitamin B12 level, later on, he developed macrocytosis and his neurological manifestations responded dramatically to injectable vitamin B12. SADSC can present with an initially normal levels of vitamin B12 [9]. Frequently, the diagnosis of cobalamine deficiency is difficult because anaemia and macrocytosis are frequently absent and cobalamine concentration is mostly borderline. However, early diagnosis and immediate therapy start are necessary to prevent irreversible neurological damage [10]. Checking the level of vitamin B12 is used to confirm the diagnosis. However, when the level of the vitamin is in the low normal range, high serum level of methyl malonic acid (MMA) and homocysteine should be used to coin the diagnosis [9]. Unfortunately, due to financial constrains we could not do the levels of the MMA and homocysteine and magnetic resonance imaging of the spinal cord. However, we suggested the diagnosis due to the rapid response to injectable vitamin B12. Another cause of such neurological manifestations in CD could be due to copper deficiency. The clinical, neuropsychological and neuro-radiological manifestations in copper deficiency myelopathy may overlap with those of sub-acute degeneration of the spinal cord due to vitamin B12 deficiency [11]. In our patient copper supplements started after the neurological improvement. Likewise, just like in our patient, other metabolic disturbances, like hypokalaemia and hypocalcaemia, may contribute to the weakness. Other neuropsychiatric complications of CD include chronic headache, migraine, peripheral neuropathy, ataxia, depression, anxiety and epilepsy with or without intracranial calcifications [12].
Figure 4. 12-year-old child with CD, 3 months on GFD, showing marked improvement. The gold standard for the diagnosis of CD had been a positive ant-tTG result and small bowl enteropathy identified on endoscopic small bowl biopsy. In 2012, the ESPGHAN suggested a non-biopsy pathway for diagnosis of CD provided the following criteria are fulfilled [13]. – Symptoms attributable to CD – Ant-tTG titer >10 ×ULN – Positive endomysial antibodies (EMA) or a second ant-tTG > 10 × ULN (if EMA is not available). – Positive HLA–DQ2 and/or HLA-DQ8 haplotype In our patient, the diagnosis of CD was based on the more recently published 2020 new guidelines for the diagnosis of paediatric CD by ESPGHAN [14]. He had an ant-tTG IgA level of 23× the ULN and a positive EMA IgA on a second sample and he showed rapid and excellent response to GFD (Figure 4). What is new in the 2020 guidelines? – For initial testing, the combination of total IgA and IgA class antibodies against transglutaminase 2 (tGT-IgA) is recommended as this is the most accurate and cost-effective, and EMA-IgA or deamidated gliadin peptide (DGP) antibodies (DGP-IgA) need not be tested initially. – The no-biopsy approach for CD diagnosis is confirmed to be safe in children with high tTG-IgA values ≥10 times the ULN with accurate, appropriate tests and positive EMA-IgA in a second serum sample. – Children with positive tTG IgA but lower titers (<10 times ULN) should undergo biopsies to decrease the risk of a false positive diagnosis. – HLA testing and the presence of symptoms are not obligatory criteria for a serology-based diagnosis without biopsies. CONCLUSIONIn this case, we reported a rare neurological presentation of CD and we highlighted the no biopsy approach for the diagnosis of paediatric CD. The case also showed the need for a high index of suspicion for the diagnosis of CD with an excellent response if the GFD is started promptly. CONFLICTS OF INTERESTThe authors declare no conflicts of interest. FUNDINGNone. ETHICAL APPROVALSigned informed consent for participation and publication of medical details and photos was obtained from the parents of this child. Ethics Committee approval was obtained from the Faculty of Medicine, University of Khartoum, Sudan. REFERENCES
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| How to Cite this Article |
| Pubmed Style Omer S.M. Suliman. Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudan J Paed. 2023; 23(1): 98-103. doi:10.24911/SJP.106-1639730602 Web Style Omer S.M. Suliman. Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. https://sudanjp.com//?mno=73248 [Access: February 05, 2026]. doi:10.24911/SJP.106-1639730602 AMA (American Medical Association) Style Omer S.M. Suliman. Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudan J Paed. 2023; 23(1): 98-103. doi:10.24911/SJP.106-1639730602 Vancouver/ICMJE Style Omer S.M. Suliman. Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudan J Paed. (2023), [cited February 05, 2026]; 23(1): 98-103. doi:10.24911/SJP.106-1639730602 Harvard Style Omer S.M. Suliman (2023) Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudan J Paed, 23 (1), 98-103. doi:10.24911/SJP.106-1639730602 Turabian Style Omer S.M. Suliman. 2023. Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudanese Journal of Paediatrics, 23 (1), 98-103. doi:10.24911/SJP.106-1639730602 Chicago Style Omer S.M. Suliman. "Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child." Sudanese Journal of Paediatrics 23 (2023), 98-103. doi:10.24911/SJP.106-1639730602 MLA (The Modern Language Association) Style Omer S.M. Suliman. "Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child." Sudanese Journal of Paediatrics 23.1 (2023), 98-103. Print. doi:10.24911/SJP.106-1639730602 APA (American Psychological Association) Style Omer S.M. Suliman (2023) Sub-acute combined degeneration of the spinal cord as first presentation of coeliac disease in a Sudanese child. Sudanese Journal of Paediatrics, 23 (1), 98-103. doi:10.24911/SJP.106-1639730602 |
Nagwa Salih, Ishag Eisa, Daresalam Ishag, Intisar Ibrahim, Sulafa Ali
Sudan J Paed. 2018; 18(1): 24-27
» Abstract » doi: 10.24911/SJP.2018.1.4
Siba Prosad Paul, Emily Natasha Kirkham, Katherine Amy Hawton, Paul Anthony Mannix
Sudan J Paed. 2018; 18(2): 5-14
» Abstract » doi: 10.24911/SJP.106-1519511375
Inaam Noureldyme Mohammed, Soad Abdalaziz Suliman, Maha A Elseed, Ahlam Abdalrhman Hamed, Mohamed Osman Babiker, Shaimaa Osman Taha
Sudan J Paed. 2018; 18(1): 48-56
» Abstract » doi: 10.24911/SJP.2018.1.7
Adnan Mahmmood Usmani; Sultan Ayoub Meo
Sudan J Paed. 2011; 11(1): 6-7
» Abstract
Mustafa Abdalla M. Salih, Mohammed Osman Swar
Sudan J Paed. 2018; 18(1): 2-5
» Abstract » doi: 10.24911/SJP.2018.1.1
Amir Babiker, Afnan Alawi, Mohsen Al Atawi, Ibrahim Al Alwan
Sudan J Paed. 2020; 20(1): 13-19
» Abstract » doi: 10.24911/SJP.106-1587138942
Bashir Abdrhman Bashir, Suhair Abdrahim Othman
Sudan J Paed. 2019; 19(2): 81-83
» Abstract » doi: 10.24911/SJP.106-1566075225
Anita Mehta, Arvind Kumar Rathi, Komal Prasad Kushwaha, Abhishek Singh
Sudan J Paed. 2018; 18(1): 39-47
» Abstract » doi: 10.24911/SJP.2018.1.6
Majid Alfadhel, Amir Babiker
Sudan J Paed. 2018; 18(1): 10-23
» Abstract » doi: 10.24911/SJP.2018.1.3
Amir Babiker, Mohammed Al Dubayee
Sudan J Paed. 2017; 17(2): 11-20
» Abstract » doi: 10.24911/SJP.2017.2.12